Respiratory Viral Diseases
DAS181 is a first-in-class host-directed recombinant sialidase protein derived from actinomyces viscosus (av). It was designed to remove sialic acids located on the surface of epithelial cells lining the human respiratory tract. These sialic acids are naturally present on cell surfaces and are needed by many viruses, including influenza, parainfluenza, metapneumovirus, and enterovirus68, for entry into cells. Cleavage of sialic acid on the patient’s respiratory epithelial cells may block viral entry, and provide a host-directed mechanism to treat respiratory viral infections.
Solid Tumors
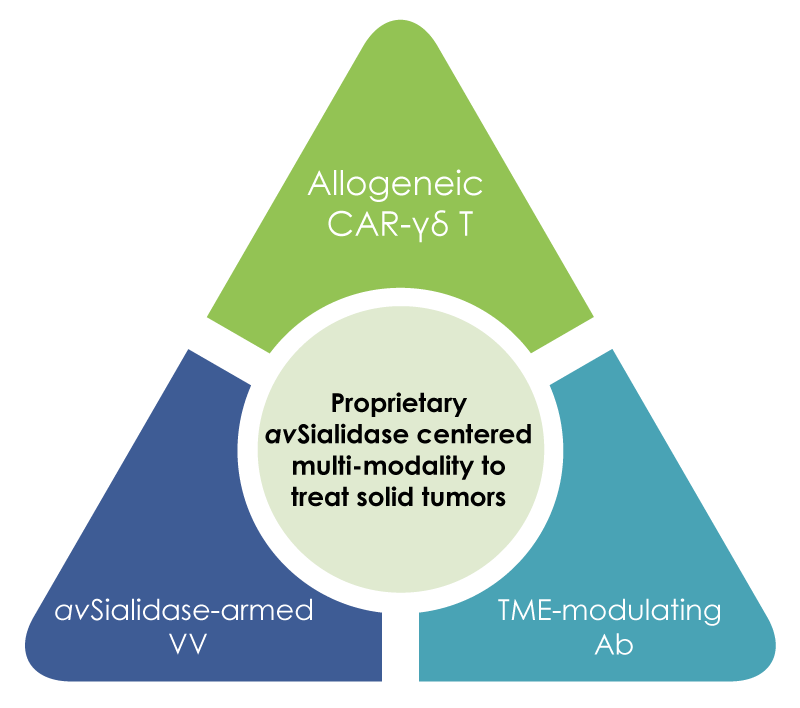
Multi-modality anti-solid tumor platforms
- Innovative therapies are required for solid tumors due to the high incidence and mortality rates they cause for cancer patients. This is especially true for tumors without driver oncogenes or tumors without a high mutational burden because these tumors do not respond to traditional targeted therapies and immune checkpoint inhibitors.
- Malignant tumors have developed multiple mechanisms, such as hyper sialylation, downregulation of MHC and upregulation of immune checkpoints to evade immune surveillance. Also, the adaptive immune system becomes progressively ineffective, as manifested by exhausted cytotoxic T cells, dysfunctional NK cells and immunosuppressive tumor-associated myeloid cells.
- To effectively prevent immune escape and restore tumor surveillance, Ansun’s approach is to target both tumor and immune cells, at different checkpoints from innate to adaptive immunity using different therapeutic modalities.